On June 11, 2021, the US Department of Health and Human Services (HHS) released revised reporting requirements for recipients of Provider Relief Fund (PRF) payments.
This announcement includes expanding the amount of time providers will have to report information, aims to reduce burdens on smaller providers, and extends key deadlines for expending PRF payments for recipients who received payments after June 30, 2020. The revised reporting requirements will be applicable to providers who received one or more payments exceeding, in the aggregate, $10,000 during a single Payment Received Period from the PRF General Distributions, Targeted Distributions, and/or Skilled Nursing Facility and Nursing Home Infection Control Distributions.
HHS began issuing notices on post-payment reporting requirements in July 2020. On January 15, 2021, HHS issued updated requirements to reflect language in the Coronavirus Response and Relief Supplemental Appropriations Act of 2021 and opened registration for the reporting portal.
The key updates to the revised reporting requirements include:
- The period of availability of funds is based on the date the payment is received (rather than requiring all payments be used by June 30, 2021, regardless of when they were received).
- Recipients are required to report for each Payment Received Period in which they received one or more payments exceeding, in the aggregate, $10,000 (rather than $10,000 cumulatively across all PRF payments).
- Recipients will have a 90-day period to complete reporting (rather than a 30-day reporting period).
- The reporting requirements are now applicable to recipients of the Skilled Nursing Facility and Nursing Home Infection Control Distribution in addition to General and other Targeted Distributions.
- The PRF Reporting Portal will open for providers to start submitting information on July 1, 2021.
The summary of reporting requirements is as follows:
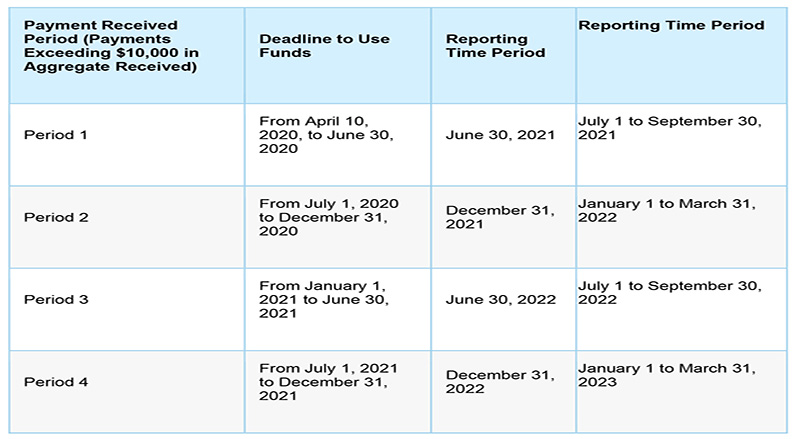
The reporting requirements do not apply to the Rural Health Clinic COVID-19 Testing Program nor the two claims reimbursements programs: the HRSA COVID-19 Uninsured Program and the HRSA COVID-19 Coverage Assistance Fund.
HRSA continues to encourage providers to establish their PRF Reporting Portal accounts at this time. Registration will also allow providers to receive updates closer to the official opening of the portal for their reporting submissions. The PRF Reporting Portal can be accessed here.
The revised reporting requirements supplanting the January 15th requirements can be accessed here.
Compliance Perspective
Issue
The federal government allocated $175 billion in payments to support healthcare providers in the battle against COVID-19 through the Provider Relief Funds. If your facility was a recipient of $10,000 or more in payment, you must register through the PRF Reporting Portal to be in compliance with the program. In addition, it is important to note that there are established deadlines by which the funds that you may have received must be used for each time period. If you do not use the funds, you risk losing the allocated funds that were provided by the federal government.
Discussion Points
- Review your policy and procedure for reporting funds received by the Provider Relief Fund, and update as necessary.
- Train appropriate staff on the reporting requirements for the Provider Relief Fund. Ensure that everyone understands that use of the funds and related documentation is being investigated by the Department of Justice (DOJ). Document that these trainings occurred and file each signed document in the employee’s individual education file.
- Audit to ensure that all reporting requirements were met by the respective deadline dates for use of the funds.


